Abstract
Background:
Cigarette smoking impairs response and outcome in pts with epidermal growth factor receptor (EGFR)-mutated advanced non-small-cell-lung cancer are treated with EGFR-TKI. Prior history of cigarette smoking might contribute to adverse outcome in pts with other types of cancer and may increase the risk of vascular events, particularly in pts treated with TKIs. The aim of this study is to evaluate the impact of cigarette smoking on outcome in pts with newly diagnosed Ph+ALL treated with the combination of intensive chemotherapy with a TKI.
Methods:
Pts with newly diagnosed Ph+ALL who received the combination of intensive therapy (hyper-CVAD) with imatinib, dasatinib, or ponatinib were analyzed. The whole population was divided into non-smoker and smoker cohorts. Smoker was defined as prior history of smoking equal to or more than 1 pack-year history of smoking before the diagnosis of Ph+ALL. Univariate and multivariate Cox proportional hazard model was used to identify prognostic factor for OS. Cumulative incidence of relapse in patients who achieve CR was assessed with death in CR as a competing risk. Gray's test was used for the comparison of cumulative incidence between cohorts.
Results:
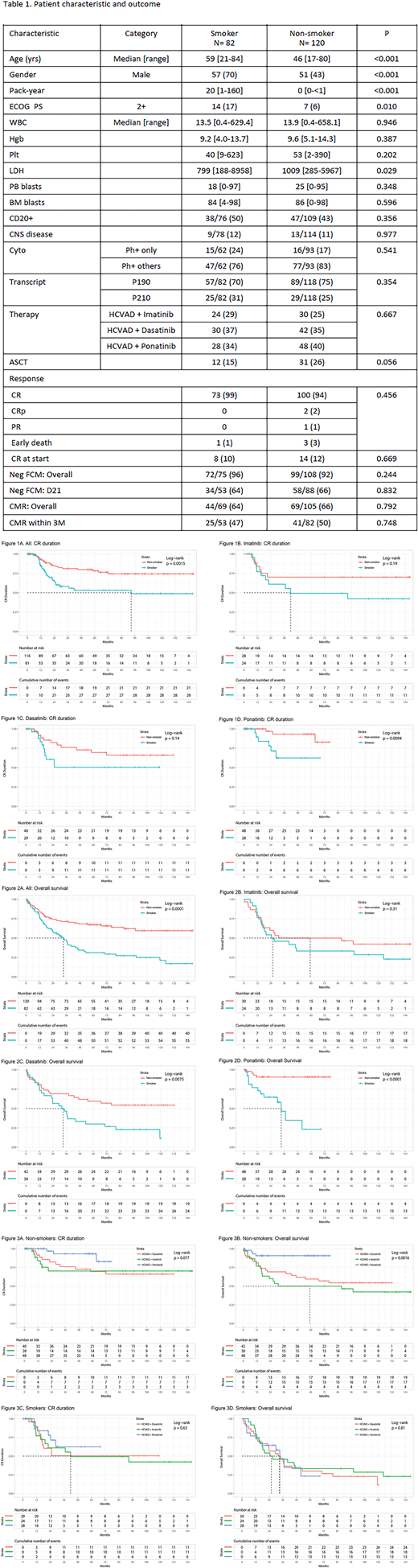
From 4/2001 to 4/2018, 202 pts with newly diagnosed Ph+ALL were analyzed including 54 pts (27%) who were treated with HCVAD + imatinib; 72 pts (36%) with HCVAD + dasatinib; and 76 pts (38%) with HCVAD + ponatinib. The median follow-up was 77 months (range, 0.2-148.9). Eighty-two pts (41%) were identified as smokers (Table 1). The median pack years of smoking was 20 pack years (range, 1-160). Smoker cohort included older pts, more males, and worse performance status than the non-smoker cohort. TKI received was similar between cohorts (p=0.667). The rates of CR, negativity of flow cytometry, and complete molecular response (CMR) were similar between cohorts. There was a tendency of higher rates of allogeneic stem cell transplant (ASCT) in the non-smoker cohort (p=0.056). Five-year CR duration was 53% and 78% in the smoker and non-smoker cohort, respectively (p=0.006) (Figure 1A); 5-year OS rate was 31% and 67%, respectively (p<0.001) (Figure 2A). Of the 54 patients who received HCVAD + imatinib, the 5-year CR duration was 49% and 70%, respectively (p=0.202) (Figure 1B); the 5-year OS rate was 33% and 50%, respectively (p=0.218) (Figure 2B). Of the 72 patients who received HCVAD + dasatinib, the 5-year CR duration was 50% and 70% in the smoker and non-smoker cohort, respectively (p=0.249) (Figure 1C); the 5-year OS rate was 30% and 59%, respectively (p=0.013) (Figure 2C). Of the 76 patients who received HCVAD + ponatinib, the 5-year CR duration was 63% and 93% in the smoker and non-smoker cohort, respectively (p=0.018) (Figure 1D); the 5-year OS rate was 23% and 91%, respectively (p<0.001) (Figure 2D). The combination of chemotherapy and ponatinib conferred a significant better outcome when compared to the combination of chemotherapy and imatinib and dasatinib among non-smokers only (CRD, p=0.077 [Figure 3A]; OS, p=0.0016 [Figure 3B]). Among smokers, no difference in outcome was observed by TKI (CRD, p=0.630 [Figure 3C]; OS, p=0.810 [Figure 3D]). Cumulative incidence of relapse was 36% and 19% in the smoker and non-smoker cohorts, respectively (p=0.027); cumulative incidence of death in CR was 31% and 17%, respectively (p=0.011). Multivariate Cox regression identified the presence of central nervous system disease (p=0.011; hazard ratio [HR], 3.141; 95% confidence interval [CI], 1.306-7.553), P190 transcript type (p=0.001; HR, 0.254; 95% CI, 0.113-0.570), the achievement of CMR within 3 months of therapy (p=0.014; HR, 2.119; 95% CI, 1.163-3.860), and smoking pack years (p=0.001; HR, 1.014; 95% CI, 1.005-1.022) as prognostic factors for OS. Each pack-year history of smoking prior to the diagnosis was associated with 1% increase of death in patients with Ph+ALL.
Conclusion: Smoking is an independent poor prognostic factor of outcome in patients treated with chemotherapy and TKI. The best outcome was obtained in non-smokers treated with chemotherapy and ponatinib (5-year OS rate of 91%). Smoking mechanisms leading to this poor outcome are being studied.
Jabbour:Novartis: Research Funding; Pfizer: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Abbvie: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding. Sasaki:Otsuka Pharmaceutical: Honoraria. Ravandi:Xencor: Research Funding; Abbvie: Research Funding; Bristol-Myers Squibb: Research Funding; Sunesis: Honoraria; Jazz: Honoraria; Amgen: Honoraria, Research Funding, Speakers Bureau; Seattle Genetics: Research Funding; Orsenix: Honoraria; Orsenix: Honoraria; Xencor: Research Funding; Astellas Pharmaceuticals: Consultancy, Honoraria; Astellas Pharmaceuticals: Consultancy, Honoraria; Amgen: Honoraria, Research Funding, Speakers Bureau; Macrogenix: Honoraria, Research Funding; Bristol-Myers Squibb: Research Funding; Seattle Genetics: Research Funding; Macrogenix: Honoraria, Research Funding; Sunesis: Honoraria; Jazz: Honoraria; Abbvie: Research Funding. Short:Takeda Oncology: Consultancy. Daver:ImmunoGen: Consultancy; Incyte: Consultancy; Sunesis: Consultancy; Alexion: Consultancy; Otsuka: Consultancy; Daiichi-Sankyo: Research Funding; Novartis: Consultancy; Novartis: Research Funding; ARIAD: Research Funding; Pfizer: Research Funding; Karyopharm: Research Funding; BMS: Research Funding; Karyopharm: Consultancy; Pfizer: Consultancy; Sunesis: Research Funding; Incyte: Research Funding; Kiromic: Research Funding. Kadia:Abbvie: Consultancy; Amgen: Consultancy, Research Funding; Novartis: Consultancy; Takeda: Consultancy; BMS: Research Funding; BMS: Research Funding; Amgen: Consultancy, Research Funding; Novartis: Consultancy; Pfizer: Consultancy, Research Funding; Takeda: Consultancy; Jazz: Consultancy, Research Funding; Abbvie: Consultancy; Jazz: Consultancy, Research Funding; Celgene: Research Funding; Pfizer: Consultancy, Research Funding; Celgene: Research Funding. Konopleva:Stemline Therapeutics: Research Funding. Jain:ADC Therapeutics: Research Funding; Genentech: Research Funding; BMS: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Infinity: Research Funding; Pharmacyclics: Research Funding; Pfizer: Research Funding; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Research Funding; Cellectis: Research Funding; Pfizer: Research Funding; Genentech: Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Research Funding; Incyte: Research Funding; ADC Therapeutics: Research Funding; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Infinity: Research Funding; Astra Zeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Cellectis: Research Funding; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Verastem: Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologioes: Research Funding; Verastem: Research Funding; BMS: Research Funding; Servier: Research Funding; Astra Zeneca: Research Funding; Celgene: Research Funding; Abbvie: Research Funding; Adaptive Biotechnologioes: Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees. Pemmaraju:Affymetrix: Research Funding; celgene: Consultancy, Honoraria; daiichi sankyo: Research Funding; abbvie: Research Funding; samus: Research Funding; SagerStrong Foundation: Research Funding; plexxikon: Research Funding; cellectis: Research Funding; stemline: Consultancy, Honoraria, Research Funding; novartis: Research Funding. Cortes:Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Astellas Pharma: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Arog: Research Funding. O'Brien:Kite Pharma: Research Funding; Abbvie: Consultancy; Pharmacyclics: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding; Sunesis: Consultancy, Research Funding; Acerta: Research Funding; Regeneron: Research Funding; Celgene: Consultancy; Astellas: Consultancy; Aptose Biosciences Inc.: Consultancy; Alexion: Consultancy; GlaxoSmithKline: Consultancy; Gilead: Consultancy, Research Funding; Vaniam Group LLC: Consultancy; Janssen: Consultancy; Pfizer: Consultancy, Research Funding; Amgen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal